Which Element Has the Smallest First Ionization Energy
67 The elements of the periodic table sorted by ionization energy. Ionization energy is the energy that an atom at ground state must be absorb to release an electron to form a cation.

Ionization Energy Ck 12 Foundation
120 rows Mar 07 2021 First Second and Third Ionization Energy eV First Ionization Energy.

. The PE Spectrum above was obtained from a neutral atom X. Which pair lists the element with the lower first ionization energy first. The tabular chart on the right is arranged by Ionization energy.
Which element has the smallest second ionization energy. 58 - Ionization energy. Which element has the smallest second ionization energy.
Answer and Explanation. For chemistry students and teachers. 60 - Covalenz radius.
First ionization energy is the energy required to separate one valence electron from an atom in gas phase. The ionization energy is minimum for cesium maximum for fluorine. Which of the following sets contain all linear molecules.
The easier the atom can release the electron the lower the energy required to remove it ie. Beryllium magnesium calcium strontium How is a jump in ionization energy related to the valence electrons of the element. Thus helium has the largest first ionization energy.
The energy increase from exterior to interior because the attraction between the. A Mg b Li c S d O e Ca 9. The lower the first ionization energy.
The first ionization energy of boron is less than that of beryllium and the first. The first chemical element is Cesium and the last one is Helium. Which of the following sets contain all linear molecules.
Potassium option C has the smallest first ionization energy from the given elements. A Mg b Li c S d O е Са 9. B HCN O2 CO2 c H2O.
The first ionization energy varies in a predictable way across the periodic table. 59 - Elements in human body. 4 rows Correspondingly which element has the smallest first ionization energy.
H The unit of ionization energy is. Which element has the smallest first ionization energy. The ionization energy decreases from top to bottom in groups and increases from left to right across a period.
Cesium has atomic number 55 and is in the fifth row of the periodic table. 4 HyS HCN CO2 6 HCN O2 CO2 c H2O CO Cl2. Based on the graph rank the group 2A elements in periods 1-5 in decreasing order of first ionization energy.
Empirical Formula of Magnesium Oxide 1. This atom can bond with Nitrogen N. Cesium has smallest ionization energy.
A Cs b Ga cK d Bi e As 8. Ionization energy is the energy that an atom at ground state must be absorb to release an electron to form a cation. A Ne B Rb C P D I E Cl Define what is meant by ionization energy and write a balanced.
A H2S HCN CO2. A Cs b Ga c K d Bi e As 8. The unit of ionization energy is.
Show your work for ALL Give the oxidation state of every atom in the following equation and state what is being oxidi. Oxygen fluorine Magnesium calcium Chlorine sulfur Helium neon. Potassium option C has the smallest first ionization energy from the given.
Cesium has smallest ionization energy. Which element has the smallest first bartleby. Of these five elements germanium Ge is closest to the bottom left-hand corner of the periodic table and would therefore have the smallest first ionization energy.
A Rb B Mg C I D As E F Select the element with the greatest metallic character. The element with the lowest ionization energy is cesium Cs. For all chemical elements the first ionization energy has a lower value.
- Elements in earthcrust. Which element has the smallest first ionization energy. D H2S CO CO2.
6 BF3 Cl2 O2 10. Which of the following elements has the smallest first ionization energy. The ionization energy is minimum for cesium maximum for fluorine.

Sulfur Element Facts Properties Production Uses Study Chemistry Electron Configuration Ionization Energy

Periodic Trends In Ionization Energy Ck 12 Foundation
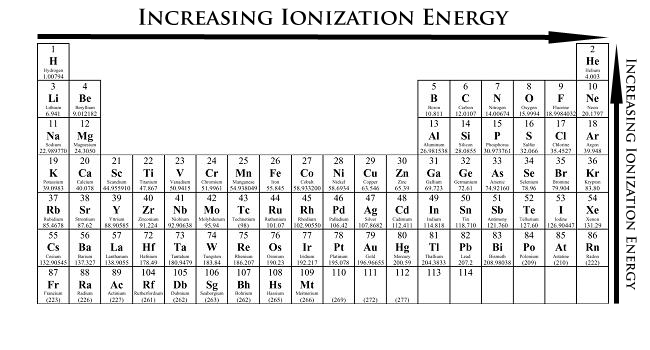
How Would You Arrange The Following Elements In Order Of Increasing Ionization Energy Te Pb Cl S Sn Socratic

Carbon Group Element Chemical Elements Britannica
Which Atom Has The Smaller Ionization Energy Be Or Mg Why Quora

Electron Affinity Of The Elements

Q Solved Which Element Requires The Least Amount Of Energy To Remove The Most Loosely Held Electron From A Gaseous Atom In The Ground State

The Parts Of The Periodic Table

Krypton Electron Configuration Ionization Energy Chemistry Basics

Solved Element Has The Lowest Ionization Energy
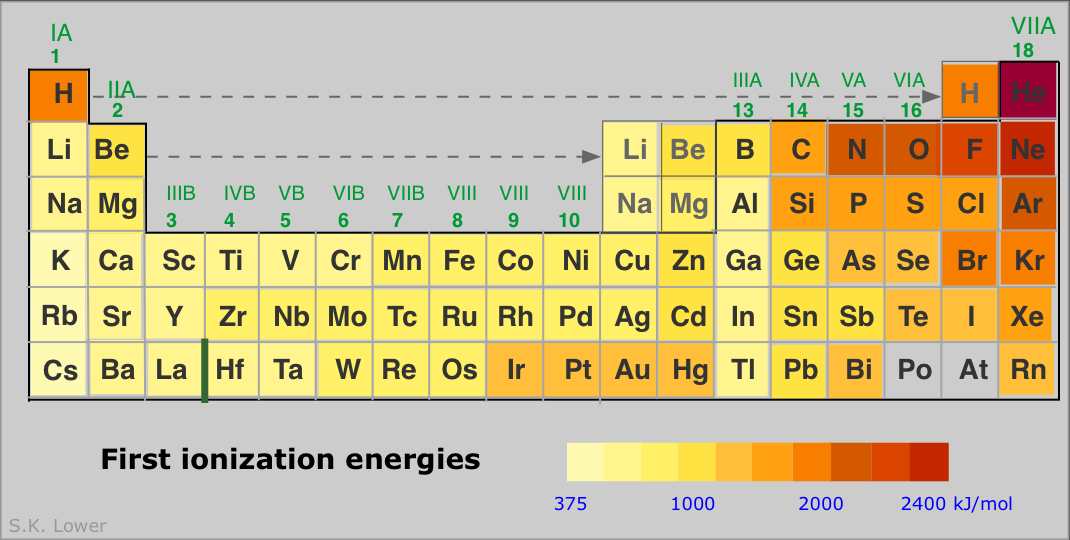
2 10 Periodic Properties Of The Elements Chemistry Libretexts
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

Group Element 14 Carbon Family Properties Trends Videos Examples
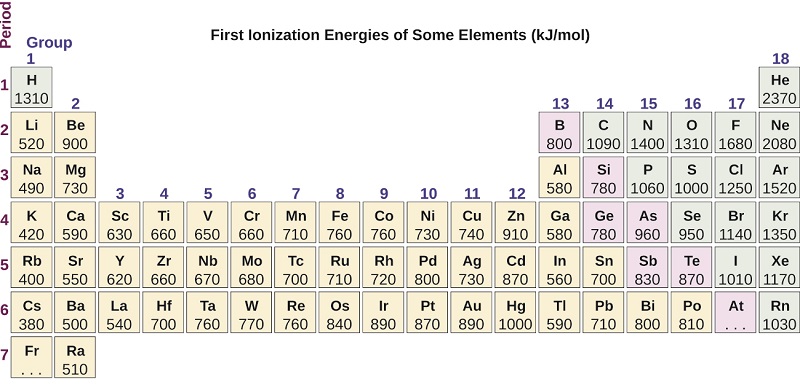
3 3 Trends In Ionization Energy Chemistry Libretexts
What Is The Order Of The Ionization Energy Of O S Br And I Quora

Which One Has The Lowest First Ionization Energy Ca P Or Ci Quora

Periodic Variations In Element Properties Chem 1305 Introductory Chemistry

Comments
Post a Comment